The lustrous patina that protects the backside of Apple's new iPad Mini isn't just some cheap shellac or sealant coat—it's actually "grown" on the metal itself in an electrochemical process known as anodization. Here's how.
Anodization takes a nonferrous metal...
Aluminum won't rust like iron and steel, but the metal is quite susceptible to corrosion. Its corrosion, or oxidation, produces a layer of aluminum oxide across the surface. Luckily, that aluminum oxide layer is actually quite durable, and it can act as a shell to protect the metal from degrading further....and coats it with an oxide layer...
The anodizing industrial process starts a controlled oxidation to create an engineered surface layer. It was pioneered in 1923, originally called as the Bengough-Stuart process as a means to seal Duralumin seaplane parts so they could resist corrosion. Today, it's used on products from roofing to cookware, along with plenty of consumer electronics....during an electrolytic process...
The anodizing process is simple. An electric current, as high as 300V DC (but normally only about 15-20V), passes through the metal. Then, the aluminum dips into an electrolytic acid bath, often composed of sulfuric acid. On the interior of the acid tank, a plate of lead (or 6063t6 alloy aluminum) acts as the cathode, or negative terminal. The charged aluminum acts as the anode, completing the circuit.In the reaction, oxygen ions migrate from the electrolyte onto the surface of the anodizing aluminum. The ions build into a protective layer of oxide that is harder, more durable, and roughly 30-percent thicker than the pure aluminum below it.
...designed to protect the metal inside.
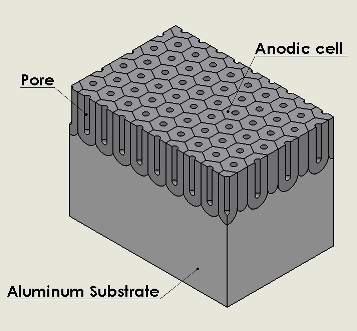
The anodized layer is highly ordered, and it contains nanopores 10-150 nanometers in diameter. These nanopores are essential in the anodizing process, as they allow oxygen to penetrate the aluminum surface and propagate the reaction. However, these pores also let water and air to penetrate the metal, causing corrosion—the exact thing anodization is meant to avoid.So the outermost layer of oxide is typically filled in with dyes, or other corrosion inhibitors, before being sealed. The thickness of the oxide layer makes a difference too. Thicker layers can absorb dyes better, while thinner layers create a brilliant sheen.
Along with corrosion resistance, anodizing can provide cosmetic benefits, and help aluminum's ability to take a coat or primer or glue. But anodizing isn't a magic bullet of metal protection. The performance trade-offs include a harder aluminum that's more brittle. The anodized layer is less likely to crack through physical wear, but it's more likely to crack through thermal heat stress.
[Anodizing - Wikipedia - Milinc]
No comments:
Post a Comment